What Is the Bond Order of S2
Reverse a string in C language. Number of electrons in bonding orbitals number of electrons in antibonding orbitals.

Inorganic Chemistry How To Find Out Unpaired Electron In S2 Molecule Chemistry Stack Exchange
Chemistry questions and answers.

. Bond order is the number of bonds between two atoms. Sulfur is a chemical element abbreviated by the symbol S and its atomic number 16. What kind of bond is sulfur.
Bond order of bonds of an atom divide by the of atom it is connected with. Bond order is defined as the number of covalent bonds between two atoms in a molecule. There is a formula for calculating bond order is.
O2 Bond Order 8- 42 42 2. It has a covalent inter molecular bond. Determine the bond order of the NO ion.
Let us first know what is meant by bond order. S2 bond order 25 S2 bond order 2 S2 bond order 15 S 2 has the weakest bond. What is the bond order of F2 and S2.
Because the nsnp energy gap increases as the nuclear charge increases the σ X 3 X. Bond order Number of electrons in bonding molecules Number of electrons in antibonding molecules2. In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons.
Most of the time bond order is equal to the number of bonds between two atoms. In resonance although the arrangement of all possible double bond locations may sometimes mean that a bond has a bond order of 15 other possibilities can occur such as 1333 1 and a third bonds. Pure elemental sulfur is a crystalline solid of bright yellow color at.
The bond order may be defined as half the difference between the number of electrons in bonding molecular orbitals Nonbonding and the number of electrons in the antibonding molecular orbital. Thats not hard at all. See the answer See the answer done loading.
We review their content and use your feedback to keep the quality high. Beside above does f2. Single bond1 double bond2 It just means the average bond number.
It comes in the form of S2 Why does S2 molecule have a double bond. In order to look at the double bond we want to find a species that has an ceO-O bond order of 2. Due to the absence of unpaired electrons in the molecular orbital of O2 it is Diamagnetic in nature.
Using MO Theory and bond order determine the number of bonds connecting the nitrogen atoms within the N 22 ion. Source We can see how close the energy the 3s and 3p atomic orbitals are because their energy separation will determine whether the π X 3 X p X x y or the σ X 3 X p X z molecular orbital is higher in energy. Similarly a triple bond may have a bond order of 25 or 2333.
Bond order number of bonding electrons - number of antibonding electrons2 If bond order 0 the two. CO2 has three atoms but the bond order of one of the two bonds is bond order two. Return to Molecular Orbital Theory Bond Order Diamagnetism Paramagnetism.
What is the bond order of S2. Experts are tested by Chegg as specialists in their subject area. It is equal to one half of the difference between the number of electrons in the bonding and antibonding molecular orbitals.
3 posts Page 1 of 1. P2 bond order 3 like N2 S2 bond order 2 like O2 Cl2 bond order 1 like F2 Cl2 has the weakest bond. Bond order is calculated by the equation.
Diatomics from the row directly above them in the periodic table. The bond order of Cl2 is 1. Bond Order ½Σ bonding e- Σ antibonding e- bo ½ σs2e σs2e 0.
Ca2 S2-What is the bond order of the OO bond in O2. Usually the higher the bond order the stronger the chemical bond. Oxygen is a diatomic molecule.
What is the bond order of Br2 s2 P2. As it has 6 valance electrons so in order o complete the. The bond order varies from one molecule to another.
Its called dioxygen ceO2 and its MO scheme is exactly the same as above except that there are two fewer electrons in the pi orbitals. Exceptions occur when the molecule contains antibonding orbitals. Bond Order is a measurement of the number of electrons involved in bonds between two elements.
Finding Bond Order Quickly. Who are the experts. Bondorder Nb Na 2 1 2Nb Na Here Nb is the number of electrons in the bonding molecular orbitals.
The molecular orbital energy-level diagram of S X 2 is given below-. NObond order 3 isoelectronic with CO Figure 513 NO bond order 25 one more antibonding electron than CO NObond order 2. Apply Molecular Orbital Theory to.
Correspondingly what is the bond order of cl2. This problem has been solved.

Inorganic Chemistry How To Find Out Unpaired Electron In S2 Molecule Chemistry Stack Exchange
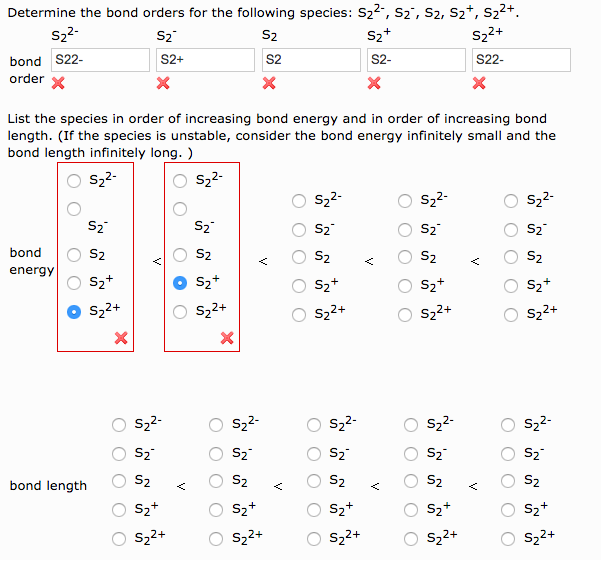
Solved S22 Szi Determine The Bond Orders For The Following Chegg Com
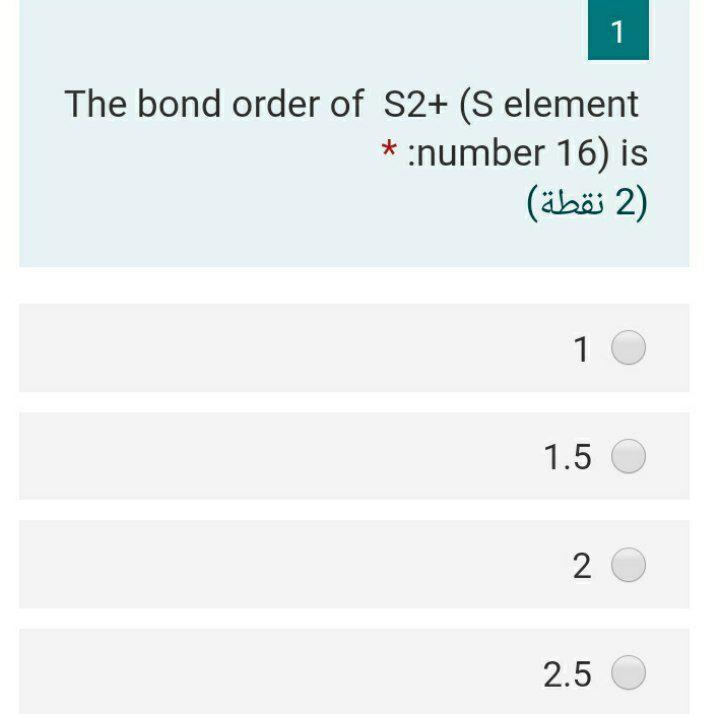
Solved 1 The Bond Order Of S2 S Element Number 16 Is 2 Chegg Com
Belum ada Komentar untuk "What Is the Bond Order of S2"
Posting Komentar